A Gem of a Battery Breakthrough
A pair of ceramic materials could unlock the potential of safer, longer-lasting solid-state batteries.
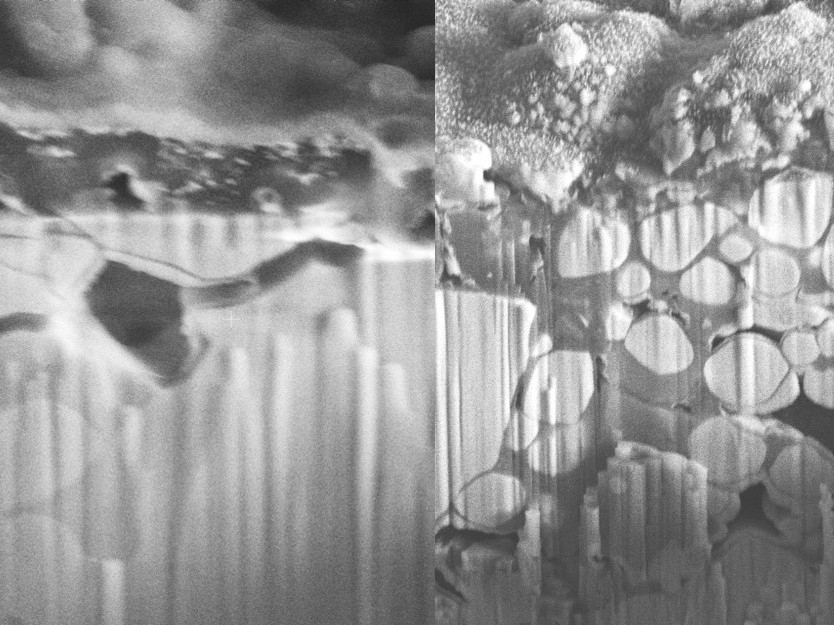
Zirconia-enhanced electrolyte (left) avoids cracking seen in the baseline sample (right).
A team of four universities and three national laboratories, led by the The University of Texas at Austin, developed a new approach for solid-state batteries, improving their performance while reducing the manufacturing costs. Solid-state batteries are an emerging energy storage technology that could unlock enhanced performance for drones, electronics and electric vehicles.
“The biggest game in town for next-generation batteries is making them all solid-state, allowing for improved safety and higher energy” said David Mitlin, professor in the Cockrell of Engineering’s Walker Department of Mechanical Engineering and the lead investigator on the new research published in Nature Materials. “However, much more work is needed before all solid-state batteries may be widely commercialized.”

David Mitlin
Today, most lithium-ion batteries use an organic liquid electrolyte, a maple-syrup-like substance that allows lithium ions to reversibly shuttle back and forth inside the battery. Despite being technologically mature, liquid electrolytes are the hydrocarbon “fuel” in the oft-reported battery fires.
Solid, ceramic-based electrolytes reduce fire risks, eliminating the hydrocarbon fuel that sustains battery thermal runaway reactions. However, ceramic electrolytes face their own hurdles, including high costs, challenging quality control during manufacturing and premature failure due to metal filament (termed dendrite) induced short circuiting.
Oxide ceramics based on the garnet structure are key materials for all solid-state batteries. Garnet’s unique structure allows lithium ions to move quickly and efficiently, making it ideal for energy storage. But even garnet has struggled to overcome the dendrite problem, which is directly linked to the formation of small cracks inside the electrolyte.
Like a jeweler refining a gemstone, the researchers have polished the garnet to reveal its full potential. Dispersing micro-scale zirconia particles throughout the garnet grains, suppresses both the cracking and the dendrites.
This method is based on carbide additives, which exothermically decompose during fabrication, inputting additional heat into the synthesis reaction. This creates an additional benefit of reducing the manufacturing cost by lowering the external temperature needed for processing.
“Zirconia really pulls double duty here,” said Yixian Wang, postdoctoral researcher in Mitlin’s lab, who is the co-lead author. “It helps densify the material while also preventing those pesky lithium dendrites from forming. It’s a win-win for battery performance and safety.”
In tests, the zirconia-modified garnet achieved nearly double the critical current density—the maximum current it can handle before short-circuiting—compared to unmodified garnet. This means batteries using this material can operate at higher power levels without compromising safety.
While battery science is the driving force of this research, the results may be applied to a wide variety of manufacturing sectors for high quality ceramics, where defect control is essential.
The full project team includes: Mitlin, Wang, Yijin Liu, Shimao Deng and Andrew Scott Manning of UT’s Materials Science and Engineering Program and Texas Materials Institute; Min Feng, Yue Qi of Brown University’s School of Engineering; Kaustubh G. Naik, Bairav S. Vishnugopi and Partha P. Mukherjee of Purdue University’s School of Mechanical Engineering; Hong Fang of Rutgers University’s Department of Physics; Puru Jena of Virginia Commonwealth University; Vikalp Raj, Noah B. Schorr, Manish Jain, Martin Salaza, Brad Boyce, Josefine D. McBrayer and Alexander M. Heusser of Sandia National Laboratories; Xiaojing Huang of Brookhaven National Laboratory; Sergiy Kalnaus of Oak Ridge National Laboratory; and John Watt of Los Alamos National Laboratory
