
As carbon capture and sequestration — the process of removing carbon dioxide from the atmosphere and storing it — gains momentum as a strategy to combat greenhouse gas emissions, scientists are exploring ways to use the captured carbon.
One promising approach is the electrochemical reduction of carbon dioxide, a technology that uses electricity to transform CO₂ into valuable fuels and chemicals. Researchers at The University of Texas at Austin have made a significant breakthrough that could accelerate this reaction and enhance its efficiency.

Joaquin Resasco, assistant professor in the Cockrell School of Engineering’s McKetta Department of Chemical Engineering
While most existing research focuses on discovering new catalyst materials to speed up the reaction, this new study highlights the importance of the micro-environment in which the reaction happens. The electrochemical reduction of CO₂ occurs in salty solutions known as electrolytes, and the type of salt used can greatly influence the process’s efficiency. By carefully selecting the right ions in the salt, the team was able to boost the rate of CO₂ conversion.
“Our research demonstrates that by controlling the electrochemical environment, we can significantly improve the efficiency of CO₂ reduction,” said Joaquin Resasco, assistant professor in the Cockrell School of Engineering’s McKetta Department of Chemical Engineering, who led the study published in Nature Catalysis. “This approach complements the search for better catalyst materials and opens up new avenues for optimizing CO₂ conversion.”
The researchers discovered that the choice of ion in the electrolyte affects the strength of the electric field at the catalyst’s surface. Smaller ions create a stronger electric field, accelerating the CO₂ conversion reaction. The team employed advanced experimental and theoretical methods, including in-situ infrared spectroscopy and ab initio molecular dynamics simulations, to understand why different ions produce varying performance levels.
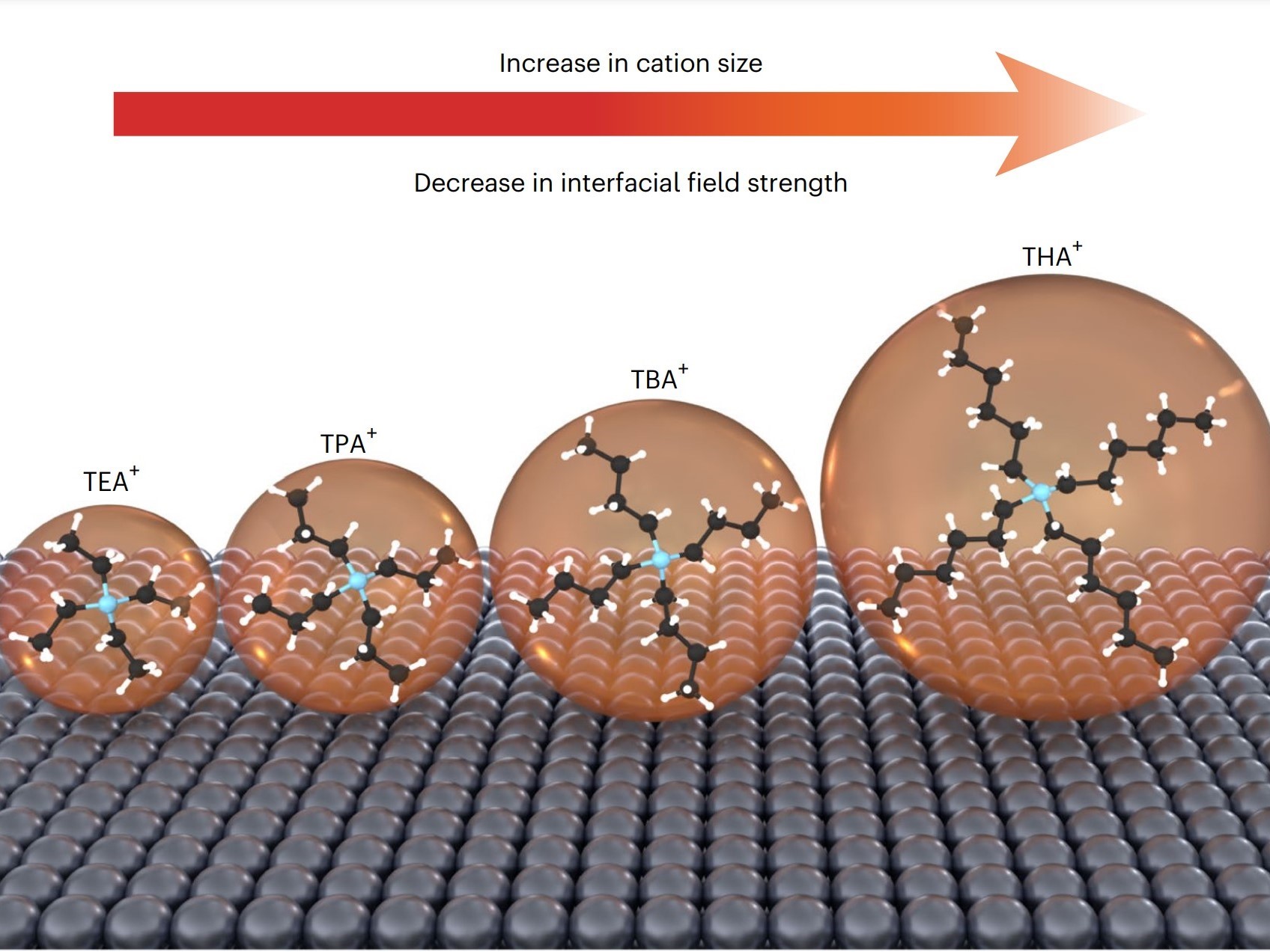
The rising concentration of CO₂ in the atmosphere is a key driver of global warming and its associated impacts, such as extreme weather events, rising sea levels, and biodiversity loss. Developing more efficient methods for reducing CO₂ is crucial for mitigating these effects and creating more sustainable and resilient energy systems.
“Scientific breakthroughs that enhance the efficiency of electrochemical processes like CO₂ conversion are important in our fight against climate change,” Resasco added. “Helping advance this science is what ultimately motivates our research.”
The research team also includes professor Joan Brennecke, Jon-Marc McGregor, Jay T. Bender, Louise Cañada of the McKetta Department of Chemical Engineering and Amanda S. Petersen and Jan Rossmeisl from the University of Copenhagen’s Department of Chemistry.
