In a new interdisciplinary study, a team of engineers led by The University of Texas at Austin have used machine learning technology in tandem with next-generation RNA sequencing to reveal the inner workings of cells and how they respond to environmental stress, particularly by focusing on RNAs, to provide new insights into biology at the molecular level.
Lydia Contreras, associate professor in the McKetta Department of Chemical Engineering in the Cockrell School of Engineering, along with collaborators from Princeton University and The Ohio State University, developed a sensing technique to allow for identification and targeting of regions within the cellular RNAs where key functions and chemical reactions occur.
Scientists now understand that RNAs are at the basis of many of the cellular decisions that change gene activity at a given time or under different stressors. This means that everything from the food we eat, to the air we breathe and the products that we consume affect how our cells and organs perform by influencing which genes are activated or remain inactive in response to environmental changes. Until now, it has been difficult to determine how RNAs in cells might interact with other key molecules that determine these processes.
“Looking at functional regions within molecules inside the cell is a new way of thinking about how biology works,” Contreras said. “In cellular biology, a lot of what is happening within the cell are condition-specific chemical reactions that do not always occur. We’re mapping those regions where important reactions that define cellular biology take place and trying to understand what triggers their activity. This is key for us to understand how living systems thrive or fail the survival of environmental changes every day.”
Through optimizing machine learning algorithms, the researchers are able to better assess local regions in key RNA molecules that could be critical to performing routine cellular functions and then observe chemical and molecular changes as they occur within the living cell.
“The collaboration between computer scientists and machine learning helped us in the high-throughput identification and targeting of regions that are most likely to host catalytic interactions in the cell, where we look at the active regions of thousands of RNA molecules at the time in a single experiment,” Contreras said. “By using data from earlier experiments, we were able to specify algorithm criteria to help us optimize these experimental designs.”
To simultaneously observe the thousands of reactions occurring locally in these molecules, the team designs molecular RNA “spying agents,” or sensors, composed of synthetic DNA plasmids that mimic the cell’s natural biology. Once injected into the cell, the plasmids manufacture RNA sensors that are chemically engineered to find and pair with most active areas of RNA molecules in the cells to determine how active mechanisms control gene activation or deactivation.
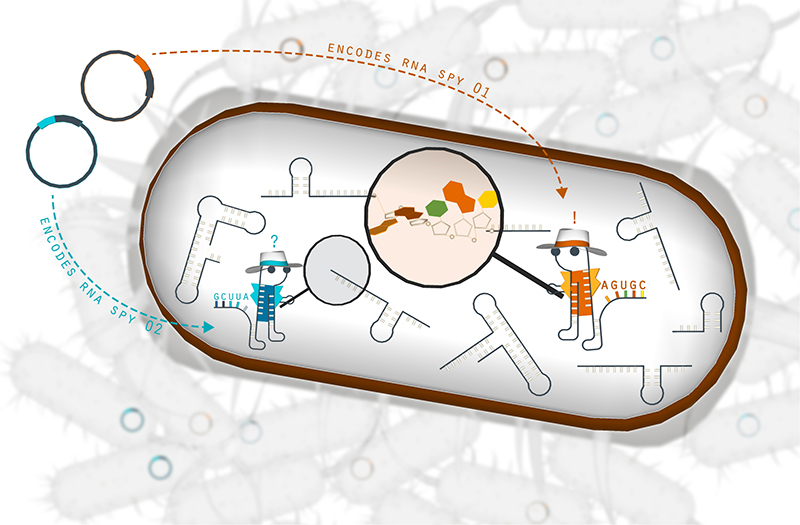
This artistic rendering from the Contreras Lab shows how molecular “spying agents” can detect accessible target RNA sections within bacterial cells.
Once a synthetic RNA sensor successfully binds to its target RNA in a specific area, the sensor unfolds, creating an elongated RNA chain that the researchers can then sequence to learn more about the status of that RNA in the cell. By controlling and varying environmental conditions during the process, the team can observe chemical and molecular changes and measure cell responses based on whether or not RNA sensors are able to pair with their target.
The team envisions that this new approach could have diverse biotechnical and medical applications that can help the broader scientific community better understand and measure cell behavior, targeted RNA therapeutic drugs or screening kits for biomedical research applications.
The team published their findings in the October issue of Nature Communications. Contreras’ research was also recently featured on KVUE News.
