Hyun Jung Kim has been developing his “gut-on-a-chip” technology for more than a decade. These miniature systems represent accurate models of the patient’s own gut, as well as the disease simulation. The aim is to use the patients’ own cells to test drugs and understand disease processes to determine the right treatment for the patient.
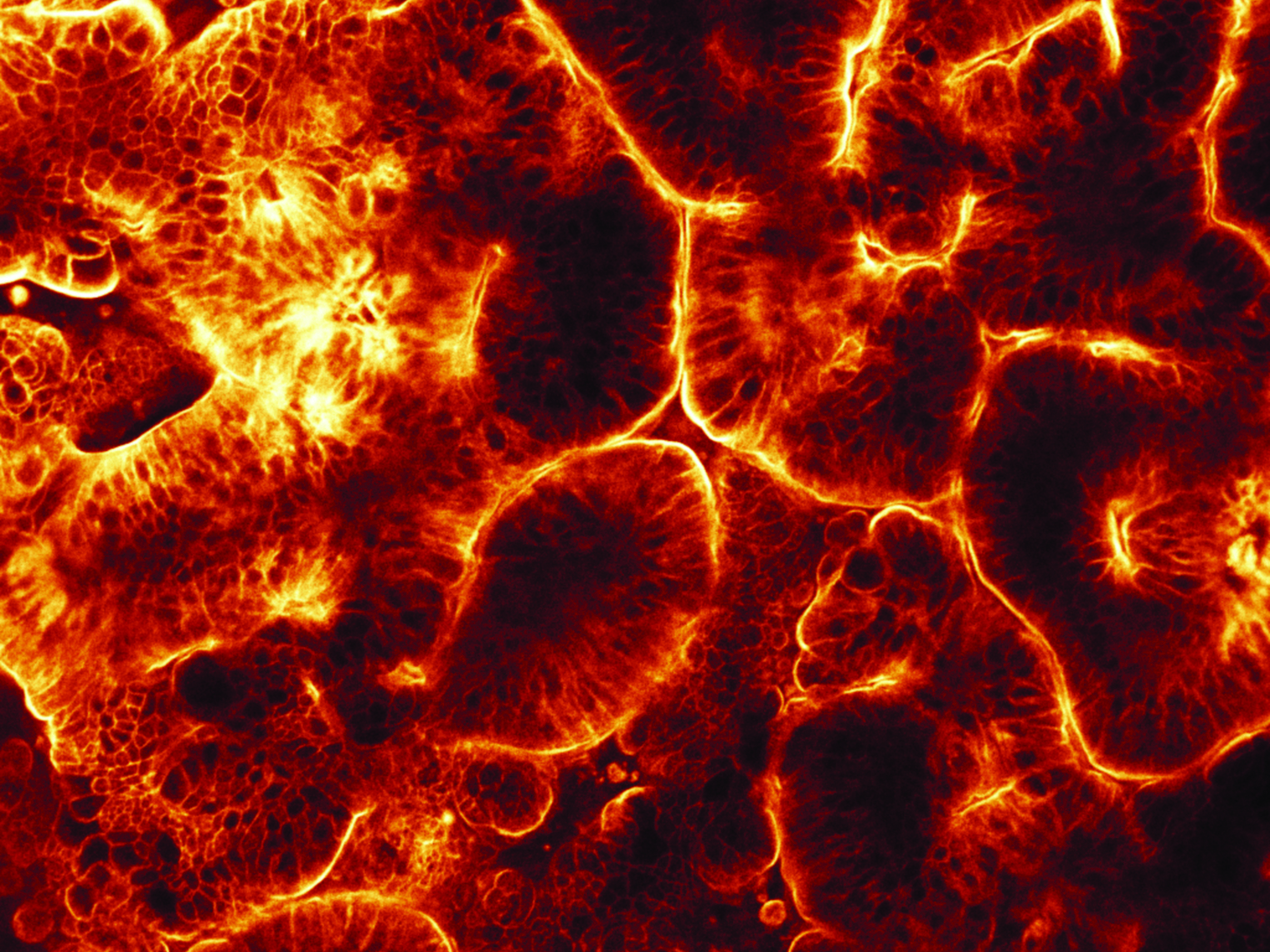
A top-view of a 3D intestinal epithelium formed in a gut-on-a-chip. Human intestinal organoids derived from biopsies of a normal donor were used as a cell source. The plasma membrane of the cells was stained with CellMask (glowing color).
Kim and Woojung Shin, an NIH/NCI F99/K00 fellow previously on Kim’s research team, published a new article in the journal Nature Protocols laying out a roadmap to generate a 3D model of the human intestinal system that can be used as part of the gut-on-a-chip technology. The researchers first discovered the underlying mechanism in 2019, and this new paper details exactly how they did it, giving others the ability to replicate for their advanced experimentation.
“The human body is such a dynamic environment,” said Kim, an assistant professor in the Cockrell School of Engineering’s Department of Biomedical Engineering. “It’s important to provide cells with the right mechanical and biological conditions to grow in a way that mimics our key organs.”
Background: In 2019, the researchers published a paper titled: Human Intestinal Morphogenesis Controlled by Transepithelial Morphogen Gradient and Flow-Dependent Physical Cues in a Microengineered Gut-on-a-Chip in iScience. In it, they were able to demonstrate the ability to grow a 3D gut that accurately mimics the shape and function of the intestinal system.
Over time, they have refined the formula and are sharing it with the larger research community in this new paper. The key to growing this system, a process known as morphogenesis, involves Wnt, which are the proteins that signal cell structures on how they should grow. The removal of a key Wnt antagonist — which inhibits their signaling capabilities — such as Dickkopf-1 (DKK-1) helped generate morphogenesis.
Why It Matters: This model of the intestinal system is much closer than anything else to mimicking the small or large intestines in our bodies. The previous gold standard used to test drugs in the pharmaceutical industry only produced a thin 2D monolayer that didn’t adequately imitate the dynamics of the intestinal system. Importantly, such conventional static models have been substantially limited to demonstrating host-microbiome interactions that can potentially orchestrate essential body functions.
A lack of a life-like testing system means the results seen in early experiments may not bear out in human trials. Gut-on-a-chip systems allow for customization as well, with the ability to simulate different race, age, diet, gender and more.
What’s Next: The researchers plan to continue to tinker with their formula to even better mimic the human body. Now that they understand the signals cells need to grow the right way, the researchers are focused on building personalized gut models as well as speeding up how quickly cells can form the 3D intestinal structures for high-throughput drug tests.
