Every living cell is made up of a massive network of proteins. Understanding everything there is to know about them can give scientists essential information about the larger processes that govern everything from how we move to how we think.
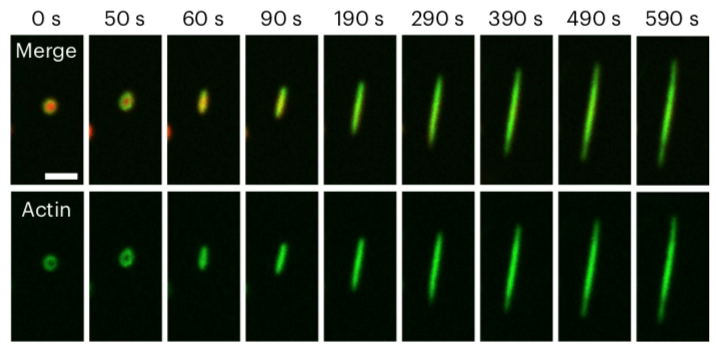
Researchers at The University of Texas at Austin and University of California San Diego just published a new paper in Nature Physics featuring previously unknown information about cellular proteins and how they organize themselves. They found that a protein called VASP phase separates into liquid droplets, which is critical for the bundling of actin filaments that help cells hold their shape and control movement.
“This discovery helps us better understand how the cytoskeleton is organized at a fundamental level, which impacts every cell in the body,” said Jeanne Stachowiak, an associate professor in the Cockrell School of Engineering’s Department of Biomedical Engineering and one of the leaders of the project. “Ultimately, by better understanding the function of the cytoskeleton, we can gain insights into virtually every known disease, the development of embryos, the aging process and more.”
The Research: The spatial organization of proteins within cells can significantly alter the cell behavior. Research in this area in recent years has led to the realization that many proteins spontaneously phase separately into liquid droplets. But what does that mean for cytoskeletal filaments and their organization?
The research shows that phase separation of VASP can lead to the formation of long bundles of actin, similar to those found in filopodia, which are actin-rich membrane antennae to help cells probe their environment.
Why It Matters: Tight regulation in the spatial organization of proteins is critical for the health of cells. Discrepancies in the spatial organization can affect key cellular processes such as cell division and immune response.
Understanding the fundamental rules that govern cytoskeletal organization establishes a foundation for further translational research in cell motility and metastasis.
The Team: The researchers credit their success to the interdisciplinary nature of the students on the team and their ability to overcome common bottlenecks. Stachowiak focuses on the biophysical systems that underlie cellular functions. Padmini Rangamani, professor in the Department of Mechanical and Aerospace Engineering at UC San Diego, focuses on biological systems design.
Several members of the team made important contributions. Kristin Graham, a graduate student in the Stachowiak group, meticulously sorted through thousands of images to carefully classify the shape of actin networks within the droplets. This also allowed the team to develop computational models explaining the underlying mechanisms. Building on top of the experimental insights, Aravind Chandrasekaran, a postdoctoral researcher in the Rangamani group, built a continuum energy model that explained the underlying scaling mechanism.
